Nearly every human malady, be it injury, infection, chronic disease, or degenerative disease, damages tissues. Moreover, 45% of all deaths can be traced to inflammation- and fibrosis-related regenerative failures. Restoring health after damage requires the answer to a key question: How can human tissues be coaxed to regenerate? Identifying instructive cues that direct refractory tissues down a regenerative path remains a critical yet elusive goal.
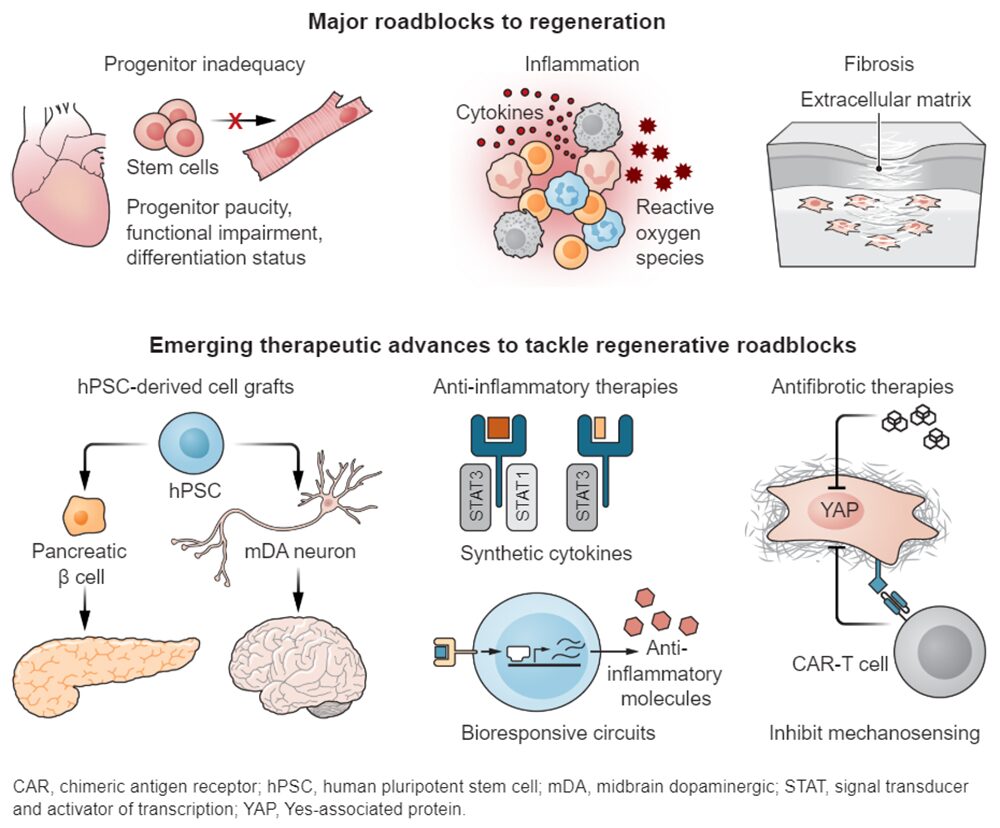
Nonetheless, approaches to target roadblocks that impede regeneration, including insufficient and/or functionally inadequate progenitor cells, fibrosis, and chronic inflammation, are continuing to progress from bench to bedside. Pivotal advances have been made to overcome these hurdles using cell therapy, in vivo reprogramming, synthetic biology, and antifibrotic and anti-inflammatory therapies, but many challenges remain and knowledge gaps must be addressed to make regeneration a mainstay of modern medicine.
The most conspicuous requirement for regenerative therapies is to replace the components of tissues that were lost or compromised by disease. Invigorating endogenous stem cells is an appealing strategy, but, to date, the greatest benefits have emerged from cell therapies. Adult stem cell–based regenerative therapies have shown clinical benefit to treat hematological malignancies, burn wounds, and ocular degeneration.
These therapeutic modalities also lend themselves to gene editing to correct monogenic perturbations. For instance, a young boy suffering from junctional epidermolysis bullosa, a lethal skin disease caused by mutations in the laminin-332 gene, was treated with an autologous (patient’s own) skin transplant. A millimeter-sized sample of the boy’s skin was collected and transduced with a retroviral vector expressing the wild-type laminin 332 cDNA; the tissue was then expanded ex vivo before being grafted to restore 80% of the body surface area.
Human pluripotent stem cell (hPSC)–based therapies have also shown promise and have entered clinical trials in the United States for type 1 diabetes (T1D; clinical trial NCT04786262), Parkinson’s disease (PD; NCT02452723 and NCT03119636), and age-related macular degeneration (AMD; NCT04339764). These three diseases are particularly amenable to stem cell–based therapies because they are associated with deficiency of a defined cell type: insulin producing pancreatic islets in T1D, midbrain dopaminergic (mDA) neurons in PD, and retinal pigment epithelial cells in AMD.
Moreover, transplantation sites are surgically accessible. Lab-generated hPSCs are being assessed for safety and efficacy in reducing disease symptoms. A case report of a PD patient who received midbrain transplant of autologous hPSC-derived mDA neurons showed stable grafting without immune reaction and exhibited improved motor function 2 years after implantation.
Graphic by K Holdski, Science